Phosphorus Electrons
Learning Objectives
- Phosphorus Electrons Count
- Phosphorus Electrons Outer Shell
- Enter The Electron Configuration For Phosphorus
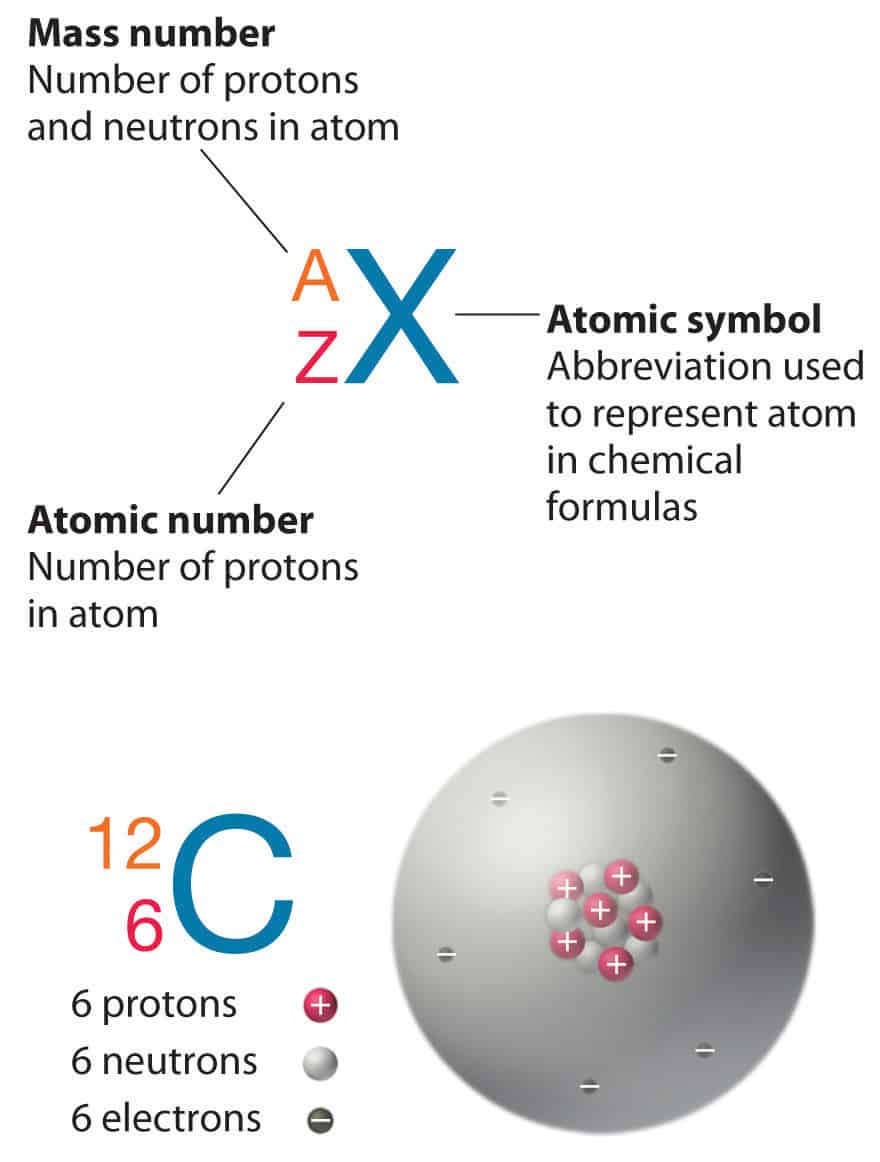
Phosphorus has an atomic number of 15 which means it has 15 protons, and when it's neutral it has 15 electrons. Electrons have a negative charge, so the 3- means that it has three more electrons. Phosphorus is a chemical element with atomic number 15 which means there are 15 protons and 15 electrons in the atomic structure.
- Compare properties of Group 15 elements.
- Explain the major application of phosphate.
- Describe the equilibria of the ionization of phosphoric acid.
Phosphorus (P) is an essential part of life as we know it. Without the phosphates in biological molecules such as ATP, ADP and DNA, we would not be alive. Phosphorus compounds can also be found in the minerals in our bones and teeth. It is a necessary part of our diet. In fact, we consume it in nearly all of the foods we eat. Phosphorus is quite reactive. This quality of the element makes it an ideal ingredient for matches because it is so flammable. Phosphorus is a vital element for plants and that is why we put phosphates in our fertilizer to help them maximize their growth.
Introduction
Phosphorus plays a big role in our existence but it can also be dangerous. When fertilizers containing phosphorus enter the water, it produces rapid algae growth. This can lead to eutrophication of lakes and rivers; i.e., the ecosystem has an increase of chemical nutrients and this can led to negative environmental effects. With all the excess phosphorus, plants grow rapidly then die, causing a lack of oxygen in the water and an overall reduction of water quality. It is thus necessary to remove excess phosphorus from our wastewater. The process of removing the phosphorus is done chemically by reacting the phosphorus with compounds such as ferric chloride, ferric sulfate, and aluminum sulfate or aluminum chlorohydrate. Phosphorus, when combined with aluminum or iron, becomes an insoluble salt. The solubility equilibrium constants of (FePO_4) and (AlPO_4) are 1.3x10-22 and 5.8x10-19, respectively. With solubility's this low, the resulting precipitates can then be filtered out.
Another example of the dangers of phosphorus is in the production of matches. The flammable nature and cheap manufacturing of white phosphorus made it possible to easily make matches around the turn of the 20th century. However, white phosphorus is highly toxic. Many workers in match factories developed brain damage and a disease called 'phosphorus necrosis of the jaw' from exposure to toxic phosphorus vapors. Excess phosphorus accumulation caused their bone tissue to die and rot away. For this reason, we now use red phosphorus or phosphorus sesquisulfide in 'safety' matches.
Discovery of Phosphorus
Named from the Greek word phosphoros ('bringer of light'), elemental Phosphorus is not found in its elemental form because this form is quite reactive. Because of this factor it took a long period of time for it to be 'discovered'. The first recorded isolation of phosphorus was by alchemist Hennig Brand in 1669 involving about 60 pails of urine. After letting a large amount of urine putrefy for a long time, Brand distilled the liquid to a paste, heated the paste, discarded the salt formed, and put the remaining substance under cold water to form solid white phosphorus. Brand's process was not very efficient; the salt he discarded actually contained most of the phosphorus. Nevertheless, he obtained some pure, elemental phosphorus for his efforts. Others of the time improved the efficiency of the process by adding sand, but still continued to discard the salt. Later, phosphorus was manufactured from bone ash. Currently, the process for manufacturing phosphorus does not involve large amounts of putrefied urine or bone ash. Instead, manufacturers use calcium phosphate and coke (Emsley).
Allotropes of Phosphorus
Phosphorus is a nonmetal, solid at room temperature, and a poor conductor of heat and electricity. Phosphorus occurs in at least 10 allotropic forms, the most common (and reactive) of which is so-called white (or yellow) phosphorus which looks like a waxy solid or plastic. It is very reactive and will spontaneously inflame in air so it is stored under water. The other common form of phosphorus is red phosphorus which is much less reactive and is one of the components on the striking surface of a match book. Red phosphorus can be converted to white phosphorus by careful heating.
White phosphorus consists of (ce{P4}) molecules, whereas the crystal structure of red phosphorus has a complicated network of bonding. White phosphorus has to be stored in water to prevent natural combustion, but red phosphorus is stable in air.
When burned, red phosphorus also forms the same oxides as those obtained in the burning of white phosphosrus, (ce{P4O6}) when air supply is limited, and (ce{P4O10}) when sufficient air is present.
Diphosphorus (P2)
Diphosphorus ((P_2)) is the gaseous form of phosphorus that is thermodynamically stable above 1200 °C and until 2000 °C. It can be generated by heating white phosphorus (see below) to 1100 K and is very reactive with a bond-dissociation energy (117 kcal/mol or 490 kJ/mol) half that of dinitrogen ((N_2)).
White Phosphorus (P4)
White phosphorus (P4) has a tetrahedral structure. It is soft and waxy, but insoluble in water. Its glow occurs as a result of its vapors slowly being oxidized by the air. It is so thermodynamically unstable that it combusts in air. It was once used in fireworks and the U.S. military still uses it in incendiary bombs.
This Youtube video link shows various experiments with white phosphorus, which help show the physical and chemical properties of it. It also shows white phosphorus combusting with air.
Red Phosphorus and Violet Phosphorus (Polymeric)
Red Phosphorus has more atoms linked together in a network than white phosphorus does, which makes it much more stable. It is not quite as flammable, but given enough energy it still reacts with air. For this reason, we now use red phosphorus in matches.
Violet phosphorus is obtained from heating and crystallizing red phosphorus in a certain way. The phosphorus forms pentagonal 'tubes'.

Black Phosphorus (Polymeric)
Black phosphorus is the most stable form; the atoms are linked together in puckered sheets, like graphite. Because of these structural similarities black phosphorus is also flaky like graphite and possesses other similar properties.
Isotopes of Phosphorus
There are many isotopes of phosphorus, only one of which is stable (31P). The rest of the isotopes are radioactive with generally very short half-lives, which vary between a few nanoseconds to a few seconds. Two of the radioactive phosphorus isotopes have longer half-lives. 32P has a half-life of 14 days and 33P has a half-life of 25 days. These half-lives are long enough to be useful for analysis and for this reason the isotopes can be used to mark DNA.
32P played an important role in the 1952 Hershey-Chase Experiment. In this experiment, Alfred Hershey and Martha Chase used radioactive isotopes of phosphorus and sulfur to determine that DNA was genetic material and not proteins. Sulfur can be found in proteins but not DNA, and phosphorus can be found in DNA but not proteins. This made Phosphorus and Sulfur effective markers of DNA and protein, respectively. The experiment was set up as follows: Hershey and Chase grew one sample of a virus in the presence of radioactive 35S and another sample of a virus in the presence of 32P. Then, they allowed both samples to infect bacteria. They blended the 35S and the 32P samples separately and centrifuged the two samples. Centrifuging separated the genetic material from the non-genetic material. The genetic material penetrated the solid that contained the bacterial cells at the bottom of the tube while the non-genetic material remained in the liquid. By analyzing their radioactive markers, Hershey and Chase found that the 32P remained with the bacteria, and the 35S remained in the supernatant liquid. These results were confirmed by further tests involving the radioactive Phosphorus..
Phosphorus and Life
We get most elements from nature in the form of minerals. In nature, phosphorus exists in the form of phosphates. Rocks containing phosphate are fluoroapatite ((ce{3Ca3(PO4)2.CaF2})), chloroapaptite, ((ce{3Ca3(PO4)2. CaCl2})), and hydroxyapatite ((ce{3Ca3(PO4)2. Ca(OH)2})). These minerals are very similar to the bones and teeth. The arrangements of atoms and ions of bones and teeth are similar to those of the phosphate containing rocks. In fact, when the (ce{OH-}) ions of the teeth are replaced by (ce{F-}), the teeth resist decay. This discovery led to a series of social and economical issues.
Nitrogen, phosphorus and potassium are key ingredients for plants, and their contents are key in all forms of fertilizers. From an industrial and economical view point, phosphorus-containing compounds are important commodities. Thus, chemistry of phosphorus has academic, commercial and industrial interests.
Chemistry of Phosphorus
As a member of the Nitrogen Family, Group 15 on the Periodic Table, Phosphorus has 5 valence shell electrons available for bonding. Its valence shell configuration is 3s23p3. Phosphorus forms mostly covalent bonds. Any phosphorus rock can be used for the production of elemental phosphorus. Crushed phosphate rocks and sand ((ce{SiO2})) react at 1700 K to give phosphorus oxide, (ce{P4O10}):
[ce{2 Ca3(PO4)2 + 6 SiO2 rightarrow P4O10 + 6 CaSiO3} label{1}]
(ce{P4O10}) can be reduced by carbon:
[ce{P4O10 + 10 C rightarrow P4 + 10 CO}. label{2}]
Waxy solids of white phosphorus are molecular crystals consisting of (ce{P4}) molecules. They have an interesting property in that they undergo spontaneous combustion in air:
[ce{P4 + 5 O2 rightarrow P4O10} label{3}]
The structure of (ce{P4}) can be understood by thinking of electronic configuration (s2p3) of (ce{P}) in bond formation. Sharing three electrons with other (ce{P}) atoms gives rise to the 6 (ce{P-P}) bonds, leaving a lone pair occupying the 4th position in a distorted tetrahedron.
When burned with insufficient oxygen, (ce{P4O6}) is formed:
[ce{P4 + 3 O2 rightarrow P4O6} label{4}]
To each of the (ce{P-P}) bonds, an (ce{O}) atom is inserted.
Burning phosphorus with excess oxygen results in the formation of (ce{P4O10}). An additional (ce{O}) atom is attached to the (ce{P}) directly:
[ce{P4 + 5 O2 rightarrow P4O10} label{5}]
Thus, the oxides (ce{P4O6}) and (ce{P4O10}) share interesting features. Oxides of phosphorus, (ce{P4O10}), dissolve in water to give phosphoric acid,
[ce{P4O10 + 6 H2O rightarrow 4 H3PO4} label{6}]
Phosphoric acid is a polyprotic acid, and it ionizes at three stages:
[ce{H3PO4 rightleftharpoons H+ + H2PO4-} label{7a}]
[ce{H2PO4- rightleftharpoons H+ + HPO4^2-} label{7b}]
[ce{HPO4^2- rightarrow H+ + PO4^3-} label{7c}]
Phosphoric Acid
Phosphoric Acid is a polyprotic acid, which makes it an ideal buffer. It gets harder and harder to separate the hydrogen from the phosphate making the pKa values increase in basicity: 2.12, 7.21, and 12.67. The conjugate bases H2PO4-, HPO42-, and PO43- can be mixed to form buffer solutions.
| Reaction | Dissociation Constant |
|---|---|
| (H_3PO_4 + H_2O rightarrow H_3O^+ + H_2PO^{4-}) | Ka1=7.5x10-3 |
| (H_2PO^{4-} + H_2O rightarrow H_3O^+ + HPO_4^{2-}) | Ka2=6.2x10-8 |
| (H_2PO^{4-} + H_2O rightarrow H_3O^+ + PO_4^{3-}) | Ka3=2.14x10-3 |
| Overall: (H_3PO_4 + 3H_2O rightarrow 3 H_3O^+ + PO_4^{3-}) |
Past and Present Uses of Phosphorus
Commercially, phosphorus compounds are used in the manufacture of phosphoric acid ((H_3PO_4)) (found in soft drinks and used in fertilizer compounding). Other compounds find applications in fireworks and, of course, phosphorescent compounds which glow in the dark. Phosphorus compounds are currently used in foods, toothpaste, baking soda, matches, pesticides, nerve gases, and fertilizers. Phosphoric acid is not only used in buffer solutions it is also a key ingredient of Coca Cola and other sodas! Phosphorus compounds were once used in detergents as a water softener until they raised concerns about pollution and eutrophication. Pure phosphorus was once prescribed as a medicine and an aphrodisiac until doctors realized it was poisonous (Emsley).
References
- Sadava, David et al. LIFE: The Science of Biology. Eighth Edition. Sinauer Associates, 2008.
- Emsley, John. The 13th Element: The Sordid Tale of Murder, Fire, and Phosphorus. John Wiley and Sons, Inc. 2000.
- Corbridge, D.E.C. The Structural Chemistry of Phosphorus. Elsevier Scientific Publishing Company. 1974.
Questions
- About 85% of the total industrial output of phosphoric acid is used
a. in the detergent industry
b. to produce buffer solutions
c. in the paint industry
d. to produce superphosphate fertilizers
e. in the manufacture of plastics - What is the product when phosphorus pentoxide (ce{P4O10}) reacts with water? Give the formula of the product.
- What is the phosphorus-containing product when (ce{PCl3}) reacts with water? Give the formula.
Solutions
- Answer... d
The middle number, (for example, 6-5-8) specifies the percentage of phosphorus compound in a fertilizer. Phosphorus is an important element for plant life.
- Answer (ce{H3PO4})
[ce{P4O10 + 6 H2O rightarrow 4 H3PO4}]
- Answer (ce{H3PO3})
[ce{PCl3 + 3 H2O rightarrow H3PO3 + 3 HCl}]
This is a weaker acid than (ce{H3PO4}).
Contributors
- Aimee Kindel (UCD), Kirenjot Grewal (UCD), Tiffany Lui (UCD)
Chung (Peter) Chieh (Professor Emeritus, Chemistry @ University of Waterloo)
How many electrons are in an atom of phosphorus?
Does phosphorus have 15 electrons?
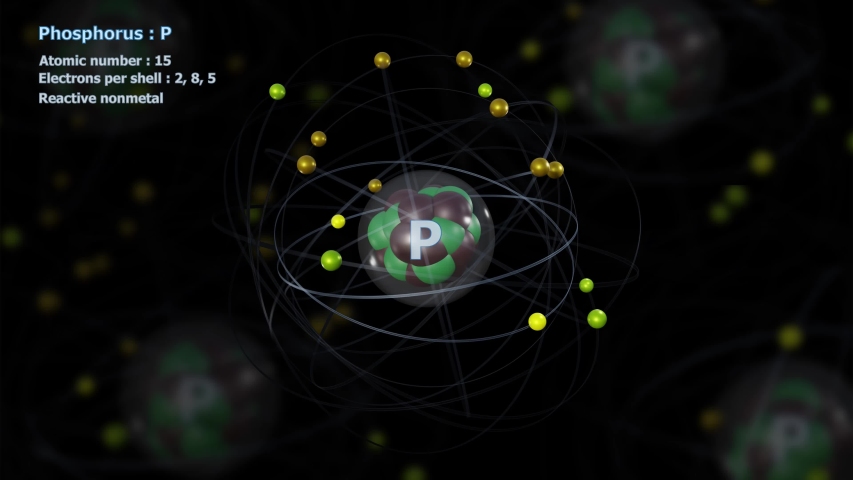
So for the element of PHOSPHORUS, you already know that the atomic number tells you the number of electrons. That means there are 15 electrons in a Phosphorus atom. Looking at the picture, you can see there are two electrons in shell one, eight in shell two, and five in shell three.
Can there be more than 8 electrons in a shell?
It is commonly used to determine the Lewis structure of molecules. However, the octet rule is not the end of the story. The main exception is the family of hypervalent molecules, in which a main group element nominally has more than 8 electrons in its valence shell.
How many electrons does phosphorus have in its valence shell?
The highest-numbered shell is the third shell, which has 2 electrons in the 3s subshell and 3 electrons in the 3p subshell. That gives a total of 5 electrons, so neutral phosphorus atoms have 5 valence electrons.
How many electrons are in the first energy level of phosphorus?
there are 2 electrons at the first energy level, and 8 at the second energy level.
How many electrons are present in a phosphorus 3+ atom?
P (Phosphorus) has an atomic number of 15. Therefore, it has 15 protons. A charge of -3 indicates that it has gained 3 electrons. Therefore, it has 18 electrons.
Phosphorus Electrons Count
How are 15 electrons arranged in a phosphorus atom?
Phosphorus Electrons Outer Shell
When we write the configuration we’ll put all 15 electrons in orbitals around the nucleus of the Phosphorus atom. In writing the electron configuration for Phosphorus the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Phosphorous go in the 2s orbital.
How does phosphorus become stable?
Because of the relatively weak intermolecular attractions (van der Waals forces) between the separate P4 molecules, the solid melts easily at 44.1 °C (111.4 °F) and boils at about 280 °C (536 °F). This may prove to be the most stable form of phosphorus, despite the relative difficulty in its preparation.
What element has 15 protons and 15 neutrons?
| Name | Phosphorus |
|---|---|
| Atomic Mass | 30.974 atomic mass units |
| Number of Protons | 15 |
| Number of Neutrons | 16 |
| Number of Electrons | 15 |
Why is 3rd shell 8 or 18?
In this sense the third shell can hold 8 electrons. In this sense the third shell can hold a total of 18 electrons. So the third shell can be considered to hold 8 or 18 electrons but in total the third shell can hold 18 electrons.
Can hold up to 2 electrons?
The general formula is that the nth shell can in principle hold up to 2 (n2) electrons. Each shell can contain only a fixed number of electrons: The first shell can hold up to two electrons, the second shell can hold up to eight ( 2 + 6) electrons, the third shell can hold up to 18 ( 2 + 6 + 10) and so on.
Can phosphorus have more than 8 valence electrons?
Unlike atoms from periods one and two that only have the s and p orbitals (total of 8 valence electrons ), atoms like phosphorus, sulfur, and chlorine can have more than 8 electrons because they are not restricted to the s and p orbitals and have a d orbital for additional electrons needed for bonding.
Enter The Electron Configuration For Phosphorus
Why does phosphorus have a valence of 5?
The valency of 5 is due to exitation of an electron from 3s orbitals to 3d orbital. It contains empty 3d orbitals so phosphorus can expand its octet by exciting on electron from 3s to 3d. Hence phosphorus can show valency 3 and 5 both.
Why is the Valency of phosphorus 3 and 5 both?
Phosphorous shows both the valencies 3 and 5. Phosphorous with valency 3: With three unpaired electrons in three p orbitals, phosphorous shows valency 3, like in case of compound PCl3. Thus, phosphorous atom undergoes sp 3 d hybridisation to form five equivalent sp3d orbitals each containing one unpaired electron.

How many electrons are in the outermost shell of phosphorus?
According to the periodic table above, phosphorus belongs to Group 5A. Therefore, Its valence electrons should be 5. The outermost orbitals, 3s2 3p3, contains 5 electrons. Thus, valence electrons for P is 5.
